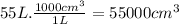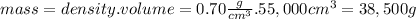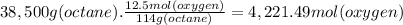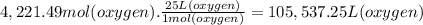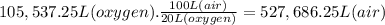Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
What volume of air is needed to burn an entire 55-l (approximately 15-gal) tank of gasoline? assume...
Questions

Chemistry, 10.07.2019 03:00

English, 10.07.2019 03:10


Mathematics, 10.07.2019 03:10

Mathematics, 10.07.2019 03:10

Chemistry, 10.07.2019 03:10








Mathematics, 10.07.2019 03:10