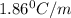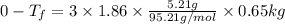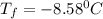Chemistry, 30.08.2019 03:10 desdes1499
If 95.21 g of mgcl2, a strong electrolyte is added to 650 g of water, how much would the freezing point of water be lowered? what would be freezing point of this solution? the kf for water is 1.86 degrees c/m.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
If 95.21 g of mgcl2, a strong electrolyte is added to 650 g of water, how much would the freezing po...
Questions






Computers and Technology, 06.09.2019 20:30




History, 06.09.2019 20:30










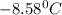
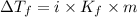
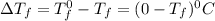 = Depression in freezing point
= Depression in freezing point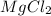 as it dissociates to give three ions.
as it dissociates to give three ions.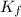 = freezing point constant =
= freezing point constant =