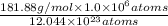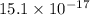Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Calculate the mass of vanadium(v) oxide (v2o5) that contains a million (1.0 *10^6) vanadium atoms. b...
Questions









Engineering, 26.10.2021 17:10

English, 26.10.2021 17:10

Chemistry, 26.10.2021 17:10


Biology, 26.10.2021 17:10





Mathematics, 26.10.2021 17:10

Health, 26.10.2021 17:10

Mathematics, 26.10.2021 17:10

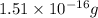
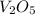 =
= 
 number of vanadium atom present in 1 moles of
number of vanadium atom present in 1 moles of 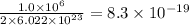 moles of
moles of 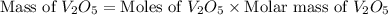
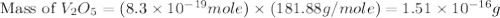
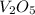 is 181.88 g/mol.
is 181.88 g/mol. atoms
atoms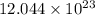 atoms
atoms vanadium atoms as follows.
vanadium atoms as follows.