

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
You know the right answer?
For a galvanic cell that uses the following two half-reactions, cr 2o 7 2-( aq) 14 h ( aq) 6 e - → 2...
Questions




Mathematics, 04.11.2020 21:20

Mathematics, 04.11.2020 21:20




English, 04.11.2020 21:20

Computers and Technology, 04.11.2020 21:20






Mathematics, 04.11.2020 21:20

Physics, 04.11.2020 21:20

Chemistry, 04.11.2020 21:20


Mathematics, 04.11.2020 21:20

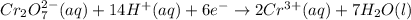
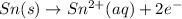
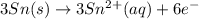
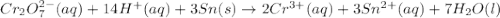
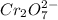 will oxidizes 3 moles of Sn
will oxidizes 3 moles of Sn moles of Sn
moles of Sn

