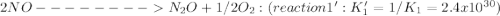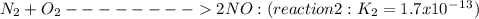Given the following: i) n20(g) 1/2 02(g) 2 no(g) ii) n2(g) 02(g) 2 no(g) ke= 4.1 x 10-31 ke= 1.7 x 10-13 find the value of the equilibrium constant for the following equilibrium reaction: n2(g) 1/202(g) n20(g) a) 7.0 x 10-44 b) 4.2 x 1017 c) 2.4 x 10-18 d) 1.6 x 109 e) 2.6 x 10-22

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
Given the following: i) n20(g) 1/2 02(g) 2 no(g) ii) n2(g) 02(g) 2 no(g) ke= 4.1 x 10-31 ke= 1.7 x...
Questions

Chemistry, 20.01.2022 07:40
















French, 20.01.2022 07:40


SAT, 20.01.2022 07:50