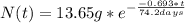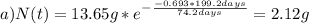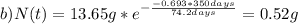Chemistry, 30.08.2019 16:10 hlgerardip4wbhx
The half-life for the radioactive decay of iridium-192 is 74.2 days. calculate the amount in grams of ir-192 that will be left from a 13.65g sample after a) 199.2 days b) 350 days

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1


Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
The half-life for the radioactive decay of iridium-192 is 74.2 days. calculate the amount in grams o...
Questions

Mathematics, 16.10.2021 19:20


Social Studies, 16.10.2021 19:20



History, 16.10.2021 19:20

English, 16.10.2021 19:20

Mathematics, 16.10.2021 19:20

Biology, 16.10.2021 19:20

Mathematics, 16.10.2021 19:20








Social Studies, 16.10.2021 19:20

Advanced Placement (AP), 16.10.2021 19:20

Mathematics, 16.10.2021 19:20

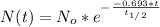
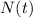 is the amount given a certain t time, and
is the amount given a certain t time, and 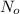 is the initial amount.
is the initial amount. 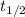 is the half life.
is the half life.