
Chemistry, 30.08.2019 18:20 jmchmom6066
A0.0035 m aqueous solution of a particular compound has ph = 2.46. the compound is (a) a weak base (b) a weak acid (c) a strong acid (d) a strong base (e) a salt

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2
You know the right answer?
A0.0035 m aqueous solution of a particular compound has ph = 2.46. the compound is (a) a weak base (...
Questions






History, 16.04.2020 18:32




Computers and Technology, 16.04.2020 18:33





History, 16.04.2020 18:33

English, 16.04.2020 18:33


English, 16.04.2020 18:33

Mathematics, 16.04.2020 18:33

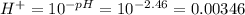
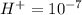 is acidic
is acidic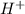 decide the behavior of the solution that is, it is acidic or basic
decide the behavior of the solution that is, it is acidic or basic

