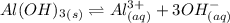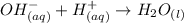Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2


Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
Consider the equilibrium of al(oh)3 (s) in water. al(oh)3 (s) = a13+ (aq) + 30h" (aq) how is the sol...
Questions

Mathematics, 28.12.2019 06:31


English, 28.12.2019 06:31


Biology, 28.12.2019 06:31

Social Studies, 28.12.2019 06:31

History, 28.12.2019 06:31

History, 28.12.2019 06:31

Mathematics, 28.12.2019 06:31





Mathematics, 28.12.2019 07:31

Mathematics, 28.12.2019 07:31



Computers and Technology, 28.12.2019 07:31

English, 28.12.2019 07:31

Computers and Technology, 28.12.2019 07:31