
Chemistry, 30.08.2019 21:10 Princess14321
The combustion of octane is given in this reaction 2c8h18++18h2 o, identify the limit reagent for this reaction with respect of carbon dioxide given starting mass of 12.85g of octane and 7.46g of oxygen.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1


You know the right answer?
The combustion of octane is given in this reaction 2c8h18++18h2 o, identify the limit reagent for th...
Questions


Arts, 03.02.2021 22:20


Mathematics, 03.02.2021 22:20


Mathematics, 03.02.2021 22:20


Mathematics, 03.02.2021 22:20

Mathematics, 03.02.2021 22:20

Mathematics, 03.02.2021 22:20



Mathematics, 03.02.2021 22:20



Mathematics, 03.02.2021 22:20



Mathematics, 03.02.2021 22:20

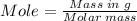
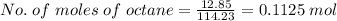
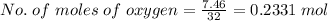
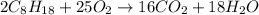
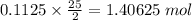 of oxygen.
of oxygen.


