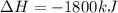Manganese metal can be obtained by the reaction of manganese dioxide with aluminium. 4al(s) + 3mnoz(s) → 2 al2o3(s) + 3 mn(s) calculate the enthalpy change of reaction. given: 2al(s) + 3/2 o2(g) →al2o3(s) ah = -1680 kj mol'. mn(s) + o2(g) → mno2(s) ah = -520 kj mol! .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
Manganese metal can be obtained by the reaction of manganese dioxide with aluminium. 4al(s) + 3mnoz(...
Questions

Mathematics, 17.10.2019 21:00

Social Studies, 17.10.2019 21:00



History, 17.10.2019 21:00


Mathematics, 17.10.2019 21:00

Physics, 17.10.2019 21:00


Biology, 17.10.2019 21:00

Mathematics, 17.10.2019 21:00

English, 17.10.2019 21:00



Mathematics, 17.10.2019 21:00

History, 17.10.2019 21:00

Mathematics, 17.10.2019 21:00

Mathematics, 17.10.2019 21:00


Mathematics, 17.10.2019 21:00

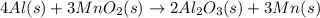
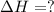
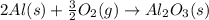
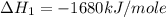
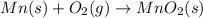
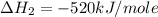
![\Delta H=[n\times \Delta H_1]+[n\times (-\Delta H_2)]](/tpl/images/0213/0280/c45d3.png)
![\Delta H=[2mole\times (-1680kJ/mole)]+[3\times -(-520kJ/mole)]](/tpl/images/0213/0280/514b6.png)