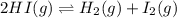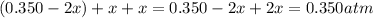Chemistry, 30.08.2019 21:30 ambinicole66
Hydrogen iodide can decompose into hydrogen and iodine gases. 2 hi(g) h2(g) + 12(g) kp for the reaction is 0.016. if 0.350 atm of hi(g) is sealed in a flask, what is the total pressure of the system when equilibrium is established? a. 0.385 atm b. 0.350 atm c. 0.279 atm d. 0.258 atm e. 0.412 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2


Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

You know the right answer?
Hydrogen iodide can decompose into hydrogen and iodine gases. 2 hi(g) h2(g) + 12(g) kp for the react...
Questions


Chemistry, 20.11.2020 23:10


Mathematics, 20.11.2020 23:10

Arts, 20.11.2020 23:10


Biology, 20.11.2020 23:10


Mathematics, 20.11.2020 23:10

Mathematics, 20.11.2020 23:10






Mathematics, 20.11.2020 23:10

English, 20.11.2020 23:10

Biology, 20.11.2020 23:10

Mathematics, 20.11.2020 23:10

History, 20.11.2020 23:10

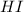 = 0.350 bar
= 0.350 bar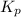 = 0.016
= 0.016