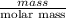Chemistry, 30.08.2019 21:30 angela6844
At 25oc, 5.77 grams of chlorine gas dissolves in enough water to give 1.0 l of solution. what is the molarity of the solution? (atomic weight: cl = 35.45).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
At 25oc, 5.77 grams of chlorine gas dissolves in enough water to give 1.0 l of solution. what is the...
Questions

Mathematics, 07.03.2020 14:49


Mathematics, 07.03.2020 14:54

Mathematics, 07.03.2020 14:55

Mathematics, 07.03.2020 14:58

Mathematics, 07.03.2020 14:58


Computers and Technology, 07.03.2020 15:10


Computers and Technology, 07.03.2020 15:11


Mathematics, 07.03.2020 15:13


Mathematics, 07.03.2020 15:26




English, 07.03.2020 15:35


Mathematics, 07.03.2020 15:40