Hydrazine, n2h4, is a weak base and is used as fuel in the space shuttle.
n2h4(aq)+h2o(l)ân2h5...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
Questions


Health, 06.05.2020 00:04



Mathematics, 06.05.2020 00:04




Mathematics, 06.05.2020 00:04





Geography, 06.05.2020 00:04

Mathematics, 06.05.2020 00:04





Physics, 06.05.2020 00:04

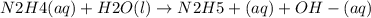
![Kb = \frac{[N2H5+][OH-]}{[N2H4]}------(1)](/tpl/images/0213/3718/63a47.png)
![[OH-] = 10^{-pOH} =10^{-3.34} =4.57*10^{-4} M](/tpl/images/0213/3718/5c095.png)
![Kb = \frac{[4.57*10^{-4}]^{2}}{[0.133]}=1.6*10^{-6}](/tpl/images/0213/3718/3b5bb.png)


