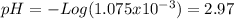Chemistry, 30.08.2019 23:30 apolloplays10
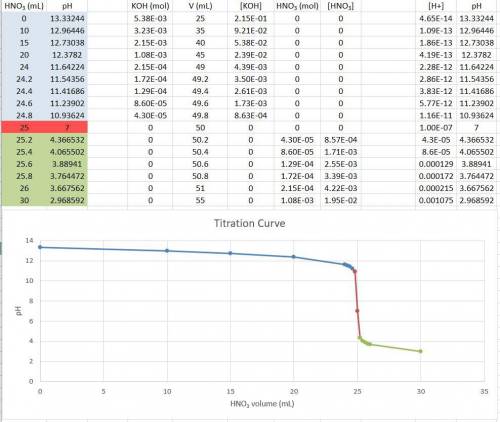
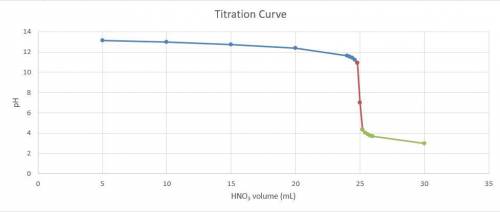
Consider the titration of a 25 ml sample of 0.215 m koh is titrated with 0.215 m hno3 a. calculate the volume of added acid that is required to reach equivalence point. (hint: what equals what at the equivalence point? ) b. the initial ph of koh c. calculate the ph after adding 5.0 ml of hno,. (hint: what kind of solution have you made? what equation can you use to calculate the ph? ) d. the ph after adding 10.0, 15.0, 20.0 ml e. the ph at the equivalence point f. the ph after adding 30 ml of hno g. sketch the titration curve. include labels for equivalence point, where there is excess oh and where there is excess h, o*.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
Consider the titration of a 25 ml sample of 0.215 m koh is titrated with 0.215 m hno3 a. calculate t...
Questions

Chemistry, 20.05.2020 05:01

History, 20.05.2020 05:01

Mathematics, 20.05.2020 05:01

Mathematics, 20.05.2020 05:01

Health, 20.05.2020 05:01

Mathematics, 20.05.2020 05:01

Physics, 20.05.2020 05:01



Chemistry, 20.05.2020 05:01

Biology, 20.05.2020 05:01


Chemistry, 20.05.2020 05:01

Mathematics, 20.05.2020 05:01




Computers and Technology, 20.05.2020 05:01

English, 20.05.2020 05:01

Mathematics, 20.05.2020 05:01

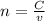
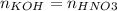
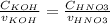
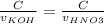
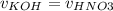
![[OH^{-} ]=\frac{moles of KOH - moles of HNO3}{total volume}](/tpl/images/0213/3384/4dd62.png)
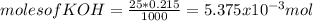
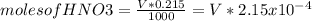
![[OH^{-} ]=\frac{5.375x10^{-3}- V*2.15x10^{-4}}{(\frac{25+v}{1000} )}](/tpl/images/0213/3384/e913a.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 5*2.15x10^{-4}}{(\frac{25+5}{1000} )}=0.143](/tpl/images/0213/3384/79893.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 10*2.15x10^{-4}}{(\frac{25+10}{1000} )}=0.0921](/tpl/images/0213/3384/e9b6e.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 15*2.15x10^{-4}}{(\frac{25+15}{1000} )}=0.0538](/tpl/images/0213/3384/ef1fb.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 20*2.15x10^{-4}}{(\frac{25+20}{1000} )}=0.0239](/tpl/images/0213/3384/6aacc.png)
![[H^{+} ]=\frac{(V-25)*2.15x10^{-4}}{\frac{V+25}{1000} }](/tpl/images/0213/3384/54d05.png)
![[H^{+} ]=\frac{(30-25)*2.15x10^{-4}}{\frac{30+25}{1000} } =1.075x10^{-3}](/tpl/images/0213/3384/583ea.png)