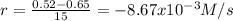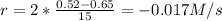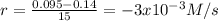a + b --> 2c

Chemistry, 30.08.2019 23:30 angsoccer02
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
concentration time data at t = 30oc
time (s) [a] (mol l-1)
0 0.65
15 0.52
30 0.35
45 0.28
60 0.22
75 0.14
90 0.095
(i) calculate the average rate of consumption of a in the first 15 seconds of reaction.
(ii) calculate the average rate of production of c in the first 15 seconds of reaction.
(iii) calculate the average rate of consumption of a in the last 15 seconds of the reaction.
(iv) explain the difference between the rates of consumption calculated in (i) and that in (iii)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
a + b --> 2c
Questions

Mathematics, 02.11.2020 23:30

World Languages, 02.11.2020 23:30


Physics, 02.11.2020 23:30

English, 02.11.2020 23:30

Mathematics, 02.11.2020 23:30

Biology, 02.11.2020 23:30






Mathematics, 02.11.2020 23:30


Mathematics, 02.11.2020 23:30

Mathematics, 02.11.2020 23:30

Mathematics, 02.11.2020 23:30


Chemistry, 02.11.2020 23:30

![r=\frac{\Delta [Concentration]}{\Delta t}](/tpl/images/0213/3224/c01cf.png)