

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2



Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
Which hybrid orbitals are used to form the sigma bonds between the carbons in acetylene? (blb ch. 9...
Questions

Mathematics, 30.03.2021 21:50






Mathematics, 30.03.2021 21:50



Mathematics, 30.03.2021 21:50

Chemistry, 30.03.2021 21:50





English, 30.03.2021 21:50




Advanced Placement (AP), 30.03.2021 21:50

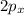 orbital and two sp hybrid orbitals are formed which are oriented at the angle of 180° . One is bonded to hydrogen and another to the carbon.
orbital and two sp hybrid orbitals are formed which are oriented at the angle of 180° . One is bonded to hydrogen and another to the carbon. 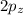 and
and 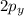 orbitals remain non-hybridized and thus are oriented perpendicularly along z and y axes respectively.
orbitals remain non-hybridized and thus are oriented perpendicularly along z and y axes respectively.

