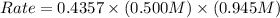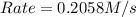Chemistry, 31.08.2019 01:00 erikamaldonado661
For the following reaction: 2a +bő20 the rate law is determined to be: rate = 0.4357 [a][b] what will be the initial rate (in m/s) if initial concentrations are: [a] = 0.500 m, [b] = 0.945 [m]

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1

Chemistry, 23.06.2019 08:40
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1
You know the right answer?
For the following reaction: 2a +bő20 the rate law is determined to be: rate = 0.4357 [a][b] what w...
Questions






Computers and Technology, 09.12.2020 15:40

Social Studies, 09.12.2020 15:40

Mathematics, 09.12.2020 15:40



Biology, 09.12.2020 15:40


Mathematics, 09.12.2020 15:40



Mathematics, 09.12.2020 15:40

Mathematics, 09.12.2020 15:40

Mathematics, 09.12.2020 15:40


English, 09.12.2020 15:40

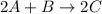
![Rate=0.4357[A][B]](/tpl/images/0213/5518/5bee5.png)