

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
Determine how many atoms of pure silver will be created when 19.83 x 1023 atoms of copper are used i...
Questions




History, 02.06.2021 22:30

Mathematics, 02.06.2021 22:30

History, 02.06.2021 22:30


Mathematics, 02.06.2021 22:30


Mathematics, 02.06.2021 22:30

History, 02.06.2021 22:30

Mathematics, 02.06.2021 22:30




English, 02.06.2021 22:30

Mathematics, 02.06.2021 22:30



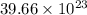
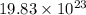
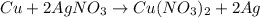
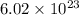 number of copper atoms react to give
number of copper atoms react to give 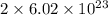 number of silver atoms.
number of silver atoms.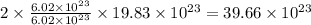 number of silver atoms.
number of silver atoms.

