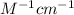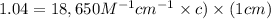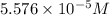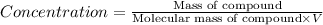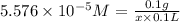Chemistry, 31.08.2019 01:30 alexisscalera7
Amolecule is known to have a molar absorptivity of 18,650 at a certain wavelength. a spectrometer is tuned to this wavelength and used to measure the absorbance of a solution of this substance in a b = 1 cm cuvette. the indicated absorbance of the sample was 1.04. the tested solution used in the experiment was prepared by dissolving 100 mg of the substance into a 100 ml volumetric flask. what is the molecular weight of the substance / molecule tested?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

You know the right answer?
Amolecule is known to have a molar absorptivity of 18,650 at a certain wavelength. a spectrometer is...
Questions


Mathematics, 23.08.2019 06:30

Mathematics, 23.08.2019 06:30




Mathematics, 23.08.2019 06:30

Mathematics, 23.08.2019 06:30

Mathematics, 23.08.2019 06:30

Mathematics, 23.08.2019 06:30

Mathematics, 23.08.2019 06:30

Biology, 23.08.2019 06:30

Social Studies, 23.08.2019 06:30


Mathematics, 23.08.2019 06:30

Geography, 23.08.2019 06:30




Advanced Placement (AP), 23.08.2019 06:30

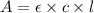
 = molar absorptivity of this solution = 18,650
= molar absorptivity of this solution = 18,650