

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
What is the mass of nickel(ii) nitrate (182.71 g/mol) dissolved in 25.0 ml of 0.100 m ni(no3)2 solut...
Questions

Mathematics, 04.02.2020 06:57




Mathematics, 04.02.2020 06:57

History, 04.02.2020 06:57

Mathematics, 04.02.2020 06:57


Biology, 04.02.2020 06:57


Mathematics, 04.02.2020 06:57

English, 04.02.2020 06:57


Biology, 04.02.2020 06:57



Mathematics, 04.02.2020 06:57

Mathematics, 04.02.2020 06:57


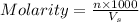
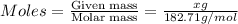
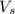 = volume of solution in ml = 25.0 ml
= volume of solution in ml = 25.0 ml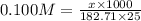
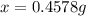
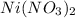 solution.
solution.

