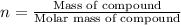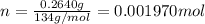Chemistry, 31.08.2019 02:30 pearpeaerrr1993
3.0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 ml of potassium permanganate to titrate it to turn pink end point. the equation for this reaction is: 5na, c,o4+ 2kmno,+ 8h, so 2mnsog+ k, so,+ 5 na, so,+ 10co2+ 8h2o a) how many moles of sodium oxalate are present in the flask?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
3.0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 ml of potassium permanganate...
Questions




History, 29.07.2020 01:01


Chemistry, 29.07.2020 01:01



Chemistry, 29.07.2020 01:01

Mathematics, 29.07.2020 01:01



Mathematics, 29.07.2020 01:01



Computers and Technology, 29.07.2020 01:01


Mathematics, 29.07.2020 01:01


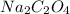 = 134 g/mol
= 134 g/mol