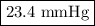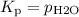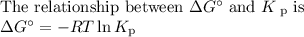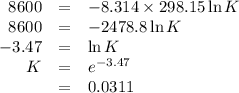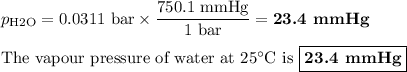Chemistry, 31.08.2019 03:10 arianna2814
Consider the reaction at 25 °c. h2o(l) ↔ h2o(g) δg° = 8.6 kj/mol calculate the pressure of water at 25 °c (hint: get k eq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
Consider the reaction at 25 °c. h2o(l) ↔ h2o(g) δg° = 8.6 kj/mol calculate the pressure of water at...
Questions


Biology, 03.05.2020 13:10








Mathematics, 03.05.2020 13:10


Mathematics, 03.05.2020 13:10

Mathematics, 03.05.2020 13:10



Geography, 03.05.2020 13:10


Mathematics, 03.05.2020 13:10

Mathematics, 03.05.2020 13:10