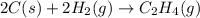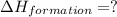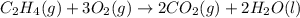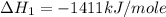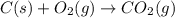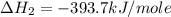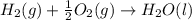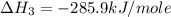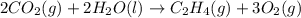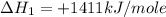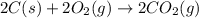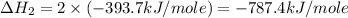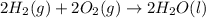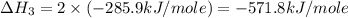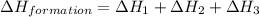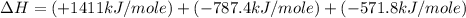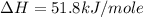Chemistry, 31.08.2019 04:10 isabella4141
How to draw hess' cycle for this question ?
carbon, hydrogen and ethane each burn exothermically in an excess of air. c(s) + o2(g) → co2(g) ahⓡ =-393.7 kj mol: 1. h2(g) + % o2(g) → h2o(1) ah® --285.9 kj mol. c2h4(g) + 302(g) → 2co2(g) + 2h2o(1) ah® --1411.0 kj mol? . use the data to calculate the standard enthalpy change of formation, ah® in kj mol? , of ethene at 298 k 2c(s) + 2h2(g) → c2h4(8)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
How to draw hess' cycle for this question ?
carbon, hydrogen and ethane each burn exothermica...
carbon, hydrogen and ethane each burn exothermica...
Questions

Advanced Placement (AP), 16.10.2019 10:30

Mathematics, 16.10.2019 10:30










Mathematics, 16.10.2019 10:30



English, 16.10.2019 10:30


Mathematics, 16.10.2019 10:30

Mathematics, 16.10.2019 10:30


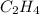 will be,
will be,