The following data was measured for the reaction above at a constant temperature.
experiment i...

Chemistry, 31.08.2019 05:30 4300224102
The following data was measured for the reaction above at a constant temperature.
experiment initial [a] mol l‐1 initial [b] mol l‐1 initial rate of formation of c (mol l‐1 s‐1)
1 0.10 0.10 1.12 x 10‐3
2 0.10 0.20 2.24 x 10‐3
3 0.20 0.10 4.48 x 10‐3
what is the order of the reaction with respect to a?
what is the order of the reaction with respect to b?
what is the rate law for this reaction?
calculate the rate constant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
Questions

English, 20.10.2020 20:01

Biology, 20.10.2020 20:01

Biology, 20.10.2020 20:01


Mathematics, 20.10.2020 20:01


English, 20.10.2020 20:01


Mathematics, 20.10.2020 20:01

English, 20.10.2020 20:01

History, 20.10.2020 20:01

Social Studies, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01


Geography, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

Business, 20.10.2020 20:01

English, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

![V=k[A]^{2} [B]](/tpl/images/0214/1245/29401.png)
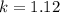
![\frac{[A]_{3}}{[A]_1{}} =2](/tpl/images/0214/1245/57061.png)
![\frac{[V]_{3}}{[V]_1{}} =4](/tpl/images/0214/1245/908c3.png)
![[A]^{2}](/tpl/images/0214/1245/66fd2.png)
![\frac{[B]_{2}}{[B]_1{}} =2](/tpl/images/0214/1245/5cc2d.png)
![\frac{[V]_{2}}{[V]_1{}} =2](/tpl/images/0214/1245/0fa6f.png)
![[B]}](/tpl/images/0214/1245/fe23b.png)
![V=k[A]^{2}[B]](/tpl/images/0214/1245/202fc.png)
![k=\frac{V}{[A]^{2}[B]}](/tpl/images/0214/1245/4243e.png)


