
Chemistry, 01.09.2019 00:30 gachaperson123
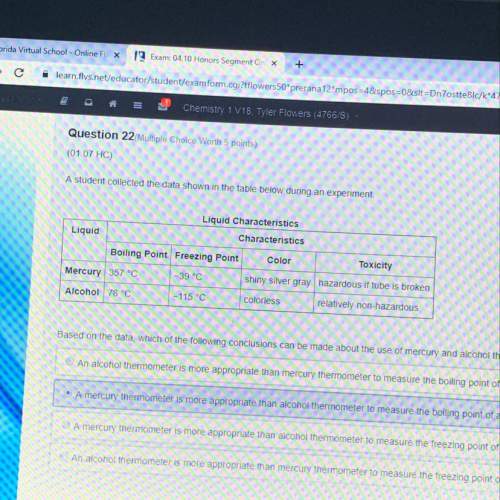
Astudent collected data shown in the table below during an experiment. based on the data, which of the following conclusions can be made about the use of mercury and alcohol thermometers
a) an alcohol thermometer is more appropriate than mercury thermometer to measure the boiling point of a colorless liquid that boils at 100°c
b) a mercury thermometer is more appropriate than an alcohol thermometer to measure the boiling point of a colorless liquid that boils at 82°c
c) a mercury thermometer is more appropriate than alcohol thermometer to measure the freezing point of a colorless liquid that freezes at -40°c
d) an alcohol thermometer is more appropriate than mercury thermometer to measure the freezing point of a colorless liquid that freezes at -20°c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
Astudent collected data shown in the table below during an experiment. based on the data, which of t...
Questions


Mathematics, 22.04.2020 19:58



Chemistry, 22.04.2020 19:58


Biology, 22.04.2020 19:58


Mathematics, 22.04.2020 19:59





History, 22.04.2020 19:59

Mathematics, 22.04.2020 19:59



Mathematics, 22.04.2020 19:59

Social Studies, 22.04.2020 19:59

Mathematics, 22.04.2020 19:59



