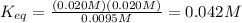Chemistry, 01.09.2019 17:20 vanleervanleer
Consider the reaction below.
at 500 k, the reaction is at equilibrium with the following concentrations.
[pci5]= 0.0095 m
[pci3] = 0.020
[ci2] = 0.020 m
what is the equilibrium constant for the given reaction?
0.042
0.42
2.4
24
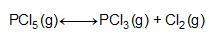

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1


Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Consider the reaction below.
at 500 k, the reaction is at equilibrium with the following conce...
at 500 k, the reaction is at equilibrium with the following conce...
Questions

Mathematics, 24.04.2020 21:47


Social Studies, 24.04.2020 21:47


Mathematics, 24.04.2020 21:47

Health, 24.04.2020 21:47



Computers and Technology, 24.04.2020 21:47

Mathematics, 24.04.2020 21:47




Spanish, 24.04.2020 21:48

Mathematics, 24.04.2020 21:48




History, 24.04.2020 21:48

![K_{eq}=\frac{[PCl_3]^1[Cl_2]^1}{[PCl_5]^1}](/tpl/images/0218/2590/d25fc.png)