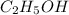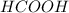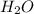Chemistry, 02.09.2019 16:10 kyleejokow
Which contains the greatest mass of oxygen: 0.75 mol of ethanol (c2h5oh), 0.60 mol of formic acid (hco2h) or 1.0 mol
of water (h2o) ? explain why.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Which contains the greatest mass of oxygen: 0.75 mol of ethanol (c2h5oh), 0.60 mol of formic acid (...
Questions



Mathematics, 22.04.2021 21:40

Mathematics, 22.04.2021 21:40






Mathematics, 22.04.2021 21:40


World Languages, 22.04.2021 21:40


Mathematics, 22.04.2021 21:40

Computers and Technology, 22.04.2021 21:40


Mathematics, 22.04.2021 21:40

Social Studies, 22.04.2021 21:40

Computers and Technology, 22.04.2021 21:40