
Chemistry, 02.09.2019 16:20 Justus4215
Areaction a(aq)+b(aq)↽−−⇀c(aq) has a standard free‑energy change of −4.20 kj/mol at 25 °c. what are the concentrations of a, b, and c at equilibrium if, at the beginning of the reaction, their concentrations are 0.30 m, 0.40 m, and 0 m, respectively?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1


Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Areaction a(aq)+b(aq)↽−−⇀c(aq) has a standard free‑energy change of −4.20 kj/mol at 25 °c. what are...
Questions

Health, 17.10.2019 03:50








Social Studies, 17.10.2019 03:50

Health, 17.10.2019 03:50

Mathematics, 17.10.2019 03:50

History, 17.10.2019 03:50


History, 17.10.2019 03:50

Physics, 17.10.2019 03:50

Mathematics, 17.10.2019 03:50



Mathematics, 17.10.2019 03:50

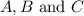 at equilibrium are 0.132 M, 0.232 M and 0.168 M respectively.
at equilibrium are 0.132 M, 0.232 M and 0.168 M respectively.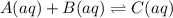
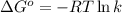
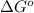 = standard free‑energy change = -4.20 kJ/mole
= standard free‑energy change = -4.20 kJ/mole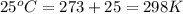
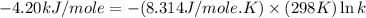
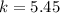
![k=\frac{[C]}{[A][B]}](/tpl/images/0220/8877/b93eb.png)
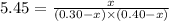
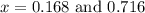
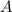 at equilibrium = (0.30-x) = 0.30 - 0.168 = 0.132 M
at equilibrium = (0.30-x) = 0.30 - 0.168 = 0.132 M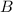 at equilibrium = (0.40-x) = 0.40 - 0.168 = 0.232 M
at equilibrium = (0.40-x) = 0.40 - 0.168 = 0.232 M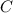 at equilibrium = x = 0.168 M
at equilibrium = x = 0.168 M


