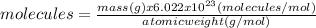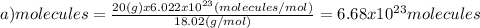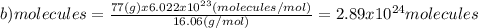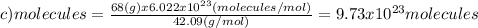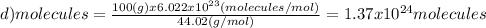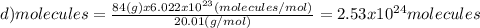Which of the following represents the least number of molecues?
(a)20.0 g of h2o (18.02g/mol)...

Chemistry, 02.09.2019 17:30 angelrgomez01
Which of the following represents the least number of molecues?
(a)20.0 g of h2o (18.02g/mol)
(b)77.0 g of ch4 (16.06 g/mol)
(c)68.0 g of cah2 (42.09 g/mol)
(d)100.0 g of n2o (44.02 g/mol)
(e)84.0 g of hf (20.01 g/mol)

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 10:30
4al + 3o2 → 2al2o3 what does the "3" in front of o2 stand for? a) it indicates that there are 5 oxygen atoms after you add the coefficient and the subscript. b) it indicates that that there are are total of 6 oxygen atoms all bonded together as a single molecule. c) it indicates that there are 3 oxygen molecules chemically bonded to each other in the reaction. d) it indicates that there are 3 separate oxygen molecules in the reaction.
Answers: 2

Chemistry, 23.06.2019 13:30
Asap a 50.0 ml soap bubble is blown in a 27.0°c room. it drifts out an open window and lands in a snow bank at -3.0°c. what is its new volume?
Answers: 1

Chemistry, 23.06.2019 15:30
Dona wrote the characteristics of two types of galaxies as shown below: type a: has a large flattened core type b: does not have a regular shape which statement is correct? type a is an irregular galaxy and type b is a lens galaxy. type a is a lens galaxy and type b is an irregular galaxy. type a is a spiral galaxy and type b is an elliptical galaxy. type a is an elliptical galaxy and type b is a spiral galaxy.
Answers: 2

Chemistry, 23.06.2019 17:00
Anonaqueous solution has a solvent that is not water. which is an example of a nonaqueous solution?
Answers: 2
You know the right answer?
Questions

History, 27.02.2020 20:25



English, 27.02.2020 20:25

History, 27.02.2020 20:25


Physics, 27.02.2020 20:25





Spanish, 27.02.2020 20:26

Mathematics, 27.02.2020 20:26




Mathematics, 27.02.2020 20:26

Mathematics, 27.02.2020 20:26

Biology, 27.02.2020 20:26

Geography, 27.02.2020 20:26