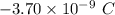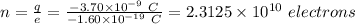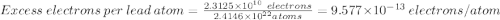Chemistry, 02.09.2019 17:30 genyjoannerubiera
Excess electrons are placed on a small lead sphere with a mass of 8.30 g so that its net charge is −3.70×10−9 c .
(a) find the number of excess electrons on the sphere.(b) how many excess electrons are there per lead atom? the atomic number of lead is 82, and its atomic mass is 207 g/mol?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
Excess electrons are placed on a small lead sphere with a mass of 8.30 g so that its net charge is −...
Questions



Chemistry, 30.01.2020 23:05

Business, 30.01.2020 23:05

Mathematics, 30.01.2020 23:05

Social Studies, 30.01.2020 23:05





Mathematics, 30.01.2020 23:05

Mathematics, 30.01.2020 23:05


History, 30.01.2020 23:05




History, 30.01.2020 23:05


Mathematics, 30.01.2020 23:05

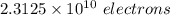
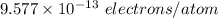
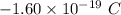 .
.