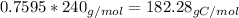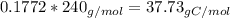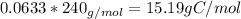Chemistry, 02.09.2019 18:10 destinyd10189
Amajor textile dye manufacturer developed new yellow dye. the dye has a percent composition of 75.9 5% c, 17.72% n
and 6.33% h by mass with the molar mass of about to 240 g/mol. determine the molecular formula of the dye.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2
You know the right answer?
Amajor textile dye manufacturer developed new yellow dye. the dye has a percent composition of 75.9...
Questions

Physics, 28.09.2020 14:01



History, 28.09.2020 14:01




Mathematics, 28.09.2020 14:01

Chemistry, 28.09.2020 14:01



English, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01




Social Studies, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Computers and Technology, 28.09.2020 14:01