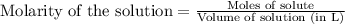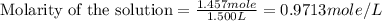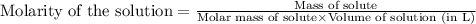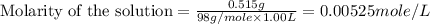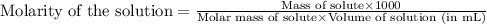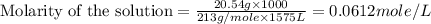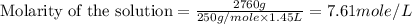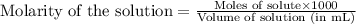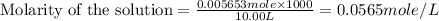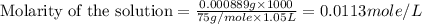Chemistry, 02.09.2019 18:20 aubreyfoster
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in 1.500 l of solution
(b) 0.515 gram ofh2so4, in 1.00 l of solution
(c) 20.54 g of al(no3)3 in 1575 ml of solution
(d)2.76 kg ofcuso4.5h2o in 1.45 l of solution
(e)0.005653 mol ofbr2 in 10.00 ml of solution
(f) 0.000889 g of glycine, c2h5no2, in 1.05 ml of solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in...
(a)1.457 mol of kcl in...
Questions



Mathematics, 04.01.2021 04:50

Mathematics, 04.01.2021 04:50

Mathematics, 04.01.2021 04:50

Mathematics, 04.01.2021 04:50




History, 04.01.2021 04:50

Mathematics, 04.01.2021 04:50




Arts, 04.01.2021 04:50


Computers and Technology, 04.01.2021 04:50

Mathematics, 04.01.2021 04:50


History, 04.01.2021 04:50

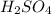 solution is, 0.00525 mole/L
solution is, 0.00525 mole/L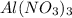 solution is, 0.0612 mole/L
solution is, 0.0612 mole/L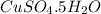 solution is, 7.61 mole/L
solution is, 7.61 mole/L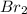 solution is, 0.0565 mole/L
solution is, 0.0565 mole/L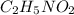 solution is, 0.0113 mole/L
solution is, 0.0113 mole/L