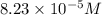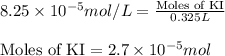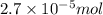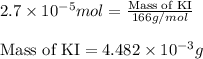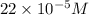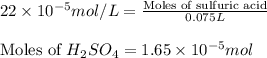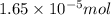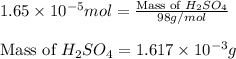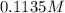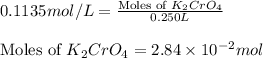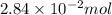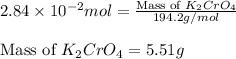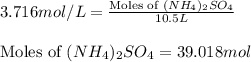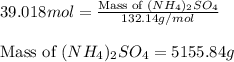Calculate the number of moles and the mass of the solute in each of the following solutions:
(a) 325 ml of 8.23 x 10-5 m kl, a source of iodine in the diet
(b) 75.0 ml of 22 x 10-5 m h2so4, a sample of acid rain
(c) 0.2500 l of 0.1135 m k2cro4 and analytical reagent used in iron assays
(d)10.5 l of 3.716 m (nh4)2so4, a liquid fertilizer

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Calculate the number of moles and the mass of the solute in each of the following solutions:
...
...
Questions

Mathematics, 09.07.2019 03:00

Mathematics, 09.07.2019 03:00

History, 09.07.2019 03:00




Mathematics, 09.07.2019 03:00



Mathematics, 09.07.2019 03:00

Chemistry, 09.07.2019 03:00

English, 09.07.2019 03:00

Mathematics, 09.07.2019 03:00


Social Studies, 09.07.2019 03:00

Mathematics, 09.07.2019 03:00



Mathematics, 09.07.2019 03:00

Biology, 09.07.2019 03:00

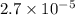 and mass is
and mass is 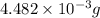
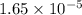 and mass is
and mass is 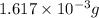
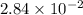 and mass is 5.51 g.
and mass is 5.51 g.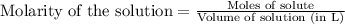 .....(1)
.....(1)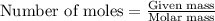 .....(2)
.....(2)