
Chemistry, 02.09.2019 18:20 endreu2005
Calculate the number of moles and the mass of the solute in each of the following solutions: (a) 2.00 l of 18.5 m h2so4, concentrated sulphuric acid
(b)100.0 ml of 3.8 x 10-5 mnacn, the minimum lethal concentration of sodium cyanide in blood serum
(c)5.50 l of 13.3 m h2co, the formaldehyde used to "fix" tissue samples.
(d)325 ml of 1.8 x 10-6 m feso4, the minimum concentration of iron sulphate detectable by taste in drinking water.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 05:30
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Calculate the number of moles and the mass of the solute in each of the following solutions: (a) 2.0...
Questions



Biology, 27.10.2020 23:50


Mathematics, 27.10.2020 23:50

Mathematics, 27.10.2020 23:50

Mathematics, 27.10.2020 23:50






Mathematics, 27.10.2020 23:50


Mathematics, 27.10.2020 23:50


English, 27.10.2020 23:50

Biology, 27.10.2020 23:50


Mathematics, 27.10.2020 23:50

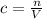
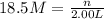
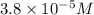 of sodium cyanide
of sodium cyanide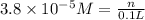
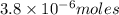 of sodium cyanide
of sodium cyanide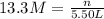
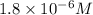 of iron sulphate
of iron sulphate 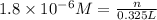
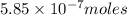 of iron sulfate.
of iron sulfate.

