
Calculate the molarity of each of the following solutions:
(a) 293 gram hcl in 666 ml of solution, a concentrated hcl solution
(b)2.026 g in 0.1250 ml of a solution used as an unknown in general chemistry laboratory
(c) 0.001 mg cd2+ in 0.100 l the maximum permissible concentration of cadmium in drinking water
(d) 0.0079 g c7h5sno3 in one ounce(29.6ml), concentration of saccharin in a diet soft drink

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Calculate the molarity of each of the following solutions:
(a) 293 gram hcl in 666 ml of solu...
(a) 293 gram hcl in 666 ml of solu...
Questions




English, 16.10.2020 16:01

Physics, 16.10.2020 16:01

Health, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01



Mathematics, 16.10.2020 16:01


History, 16.10.2020 16:01

Biology, 16.10.2020 16:01

Arts, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01

English, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01

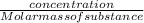
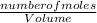
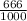 dm³ , 0.666dm³
dm³ , 0.666dm³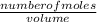
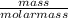
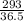 = 8.03mole
= 8.03mole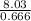 = 12.06moldm⁻³
= 12.06moldm⁻³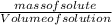 =
= 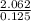 = 16.496g/mL
= 16.496g/mL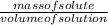 =
= 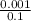 = 0.01mg/L
= 0.01mg/L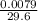 = 0.00027g/mL
= 0.00027g/mL

