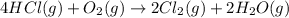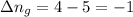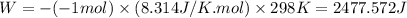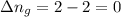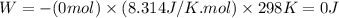Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
11. assuming that the gases are ideal, calculate the amount of work done (joules) in each of the fol...
Questions

Mathematics, 16.10.2019 07:20



Physics, 16.10.2019 07:20



History, 16.10.2019 07:20

Social Studies, 16.10.2019 07:20


Mathematics, 16.10.2019 07:20

Chemistry, 16.10.2019 07:20





Mathematics, 16.10.2019 07:20



Mathematics, 16.10.2019 07:20

Mathematics, 16.10.2019 07:20

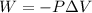
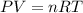
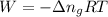 ......(1)
......(1)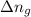 = difference in number of moles of products and reactants =
= difference in number of moles of products and reactants = 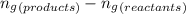
![25^oC=[273+25]K=298K](/tpl/images/0221/2455/0e82f.png)