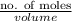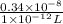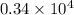Chemistry, 02.09.2019 19:30 elijahjacksonrp6z2o7
The level of mercury in a stream was suspected to be above the minimum considered safe (1part per million by weight). an analysis indicated that the concentration was 0.68 parts per million .assume a density of 1.0 g/ml and calculate the molarity of mercury is the stream.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
The level of mercury in a stream was suspected to be above the minimum considered safe (1part per mi...
Questions

Social Studies, 09.10.2019 14:10

History, 09.10.2019 14:10



Chemistry, 09.10.2019 14:10

Mathematics, 09.10.2019 14:10


Mathematics, 09.10.2019 14:10



Biology, 09.10.2019 14:10

Biology, 09.10.2019 14:10


Geography, 09.10.2019 14:10


Chemistry, 09.10.2019 14:10

Geography, 09.10.2019 14:10

History, 09.10.2019 14:10

Physics, 09.10.2019 14:10

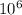 mg of solution.
mg of solution.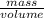
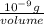
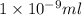 (as 1 mg = 0.001 g)
(as 1 mg = 0.001 g)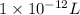 .
.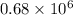 g
g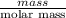
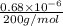
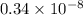 mol
mol