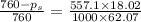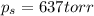Chemistry, 02.09.2019 20:20 genyjoannerubiera
Calculate the vapor pressure lowering of a solution at 100.0 °c that contains 557.1 g of ethylene glycol (molar mass g/mol). the vapor pressure of pure water at t00.0 °c is 760 torr. (2 points) 62.07 g/mol) in 1000.0 g of water (h20 molar mass 18.02 a) 106 torr b) 0.756 torr c) 760 torr (d)186 torr e) none of these

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 03:40
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Calculate the vapor pressure lowering of a solution at 100.0 °c that contains 557.1 g of ethylene gl...
Questions



English, 03.11.2019 19:31

English, 03.11.2019 19:31


Mathematics, 03.11.2019 19:31

Mathematics, 03.11.2019 19:31

Mathematics, 03.11.2019 19:31



Mathematics, 03.11.2019 19:31

Social Studies, 03.11.2019 19:31

English, 03.11.2019 19:31


History, 03.11.2019 19:31


Social Studies, 03.11.2019 19:31


Mathematics, 03.11.2019 19:31

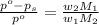
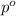 = vapor pressure of pure solvent (water) = 760 torr
= vapor pressure of pure solvent (water) = 760 torr 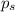 = vapor pressure of solution = ?
= vapor pressure of solution = ?
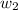 = mass of solute (ethylene glycol) = 557.1 g
= mass of solute (ethylene glycol) = 557.1 g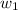 = mass of solvent (water) = 1000.0 g
= mass of solvent (water) = 1000.0 g
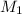 = molar mass of solvent (water) = 18.02 g/mole
= molar mass of solvent (water) = 18.02 g/mole
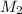 = molar mass of solute (ethylene glycol) = 62.07 g/mole
= molar mass of solute (ethylene glycol) = 62.07 g/mole