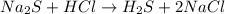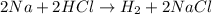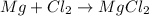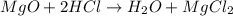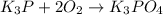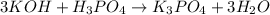Chemistry, 02.09.2019 20:30 makaylarae8781
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl -> h2s + 2nacl
(b)2na + 2hcl -> h2 + 2nacl
(c)mg + cl2 = mgcl2
(d)mgo + 2hcl = h2o + mgcl2
(e)k3p+2o2 -> k3po4
(f)3koh +h3po4 -> k3po4 + 3h2o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl...
(a)na2s + hcl...
Questions


Computers and Technology, 14.03.2020 02:44






History, 14.03.2020 02:44

Mathematics, 14.03.2020 02:44

Mathematics, 14.03.2020 02:44



Mathematics, 14.03.2020 02:44

Mathematics, 14.03.2020 02:44

Computers and Technology, 14.03.2020 02:44



English, 14.03.2020 02:45