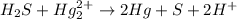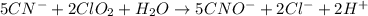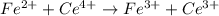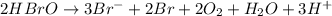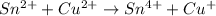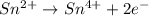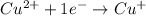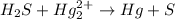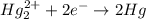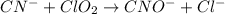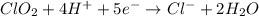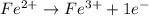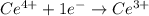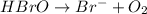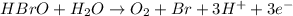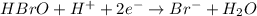Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2


Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
You know the right answer?
Balance each of the following equations according to the half- reaction method:
(a) sn2+ + cu...
(a) sn2+ + cu...
Questions



Mathematics, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00

Arts, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00



Social Studies, 06.10.2021 14:00


Arts, 06.10.2021 14:00


English, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00


English, 06.10.2021 14:00

English, 06.10.2021 14:00