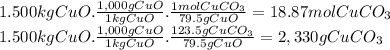Chemistry, 02.09.2019 22:10 BlueExorcistReaper
Determine the number of moles and mass requested for each reaction in exercise 4.42.
refer to exercise 4.42.
write the balanced equation, then outline the steps necessary determine the information equation in each of the following:
(a) the number of moles than the mass of the chlorine cl2 required to react with 10.0 g of sodium metal na to produce sodium chloride nacl
(b) the number of moles and the mass of the oxygen formed by the decomposition of 1.252 gram of mercury (ii) oxide
(c)the number of the moles and the mass of the sodium nitrate nano3 required to produce 128 gram of oxygen (nano2 is the other product)
(d)the number of moles and the mass of the carbon dioxide formed by the combustion of 20.0 kg of carbon in excess of oxygen
(e)the number of moles and the mass of the copper(ii) carbonate needed to produce 1.500 kg of copper ii oxide (co2 is the other product)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
Determine the number of moles and mass requested for each reaction in exercise 4.42.
refer to...
refer to...
Questions








Computers and Technology, 11.02.2020 19:37







Arts, 11.02.2020 19:38




Mathematics, 11.02.2020 19:38

Physics, 11.02.2020 19:38

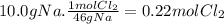
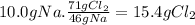
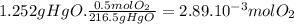
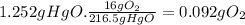
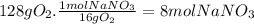
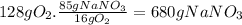
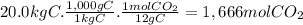
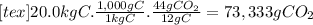 " alt="20.0kgC.\frac{1,000gC}{1kgC} .\frac{44gCO_{2}}{12gC} =73,333gCO_{2" />" />
" alt="20.0kgC.\frac{1,000gC}{1kgC} .\frac{44gCO_{2}}{12gC} =73,333gCO_{2" />" />