

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1


Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

You know the right answer?
Lead(ii) nitrate is added slowly to a solution that is 0.0800 m in ct ions. calculate the concentrat...
Questions

Biology, 17.04.2020 01:58



Mathematics, 17.04.2020 01:58


History, 17.04.2020 01:58

Spanish, 17.04.2020 01:58

Mathematics, 17.04.2020 01:58




Mathematics, 17.04.2020 01:58








 ion is 0.0375 M.
ion is 0.0375 M.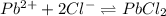
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0221/4126/7fd11.png)
![2.40\times 10^{-4}=[Pb^{2+}]\times (0.0800)^2](/tpl/images/0221/4126/e30e6.png)
![[Pb^{2+}]=0.0375M](/tpl/images/0221/4126/560e3.png)


