
Chemistry, 02.09.2019 22:20 Jackiecroce12
H2 is produced by the reaction of 118.5 ml of a 0.8775-m solution of h3po4 according to the following equations: 2cr +
2h3po4 ? 3h2 + 2crpo4.
(a) outline the steps neccessary to determine the number of moles and mass of h2
(b)perform the calculations outlined

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2


Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
H2 is produced by the reaction of 118.5 ml of a 0.8775-m solution of h3po4 according to the followin...
Questions

English, 24.03.2021 17:10


Mathematics, 24.03.2021 17:10


Mathematics, 24.03.2021 17:10



Biology, 24.03.2021 17:10

Mathematics, 24.03.2021 17:10

Spanish, 24.03.2021 17:10

Chemistry, 24.03.2021 17:10

Mathematics, 24.03.2021 17:10

Arts, 24.03.2021 17:10

Mathematics, 24.03.2021 17:10

Mathematics, 24.03.2021 17:10

Mathematics, 24.03.2021 17:10




Mathematics, 24.03.2021 17:10

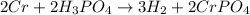
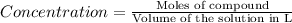
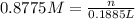
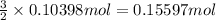 of hydrogen gas
of hydrogen gas

