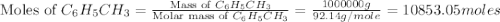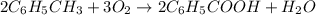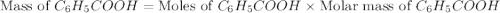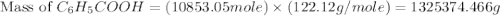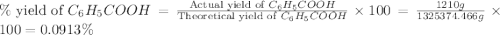Chemistry, 02.09.2019 23:20 aide1234564
Toulene, c6h5ch3 is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h, which is used to prepare the food perservative sodium benzoate, c6h5co2na. what is the percent yield os a reaction that converts 1000 kg of toulene to 1.21 kg of benzoic acid
2c6 h5 ch3 + 3o2 ? 2c6 h5 co2 h + 2h2o

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the h+ concentration for an aqueous solution with poh = 4.01 at 25 ∘c? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1


You know the right answer?
Toulene, c6h5ch3 is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h,...
Questions





Spanish, 10.12.2020 09:20



Mathematics, 10.12.2020 09:20



Mathematics, 10.12.2020 09:20

Mathematics, 10.12.2020 09:20

Mathematics, 10.12.2020 09:20

History, 10.12.2020 09:20

Mathematics, 10.12.2020 09:20

Mathematics, 10.12.2020 09:20

Mathematics, 10.12.2020 09:20


English, 10.12.2020 09:20

Mathematics, 10.12.2020 09:20

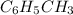 = 1000 kg = 1000000 g
= 1000 kg = 1000000 g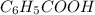 = 122.12 g/mole
= 122.12 g/mole