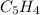Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
The principal component of mothballs is naphthalene, a compound with the molecular mass of about 130...
Questions


Mathematics, 28.07.2019 06:00

Mathematics, 28.07.2019 06:00

History, 28.07.2019 06:00

Mathematics, 28.07.2019 06:00


Mathematics, 28.07.2019 06:00

History, 28.07.2019 06:00


English, 28.07.2019 06:00

Business, 28.07.2019 06:00


History, 28.07.2019 06:00


Mathematics, 28.07.2019 06:00

Spanish, 28.07.2019 06:00




Mathematics, 28.07.2019 06:00

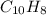 - Molecular formula
- Molecular formula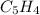 - Empirical formula
- Empirical formula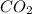 = 10.3 × 10⁻³ g /44.01 g/mol = 0.2340×10⁻³ moles
= 10.3 × 10⁻³ g /44.01 g/mol = 0.2340×10⁻³ moles