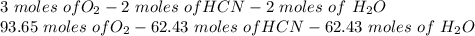Hydrogen cyanide is produced industrially from the reaction of gaseous ammonia, oxygen, and methane: 2nh3 1g2 1 3o2 1g2 1 2ch4 1g2 h 2hcn1g2 1 6h2o1g2 if 5.00 3 103 kg each of nh3, o2, and ch4 are reacted, what mass of hcn and of h2o will be produced, assuming 100% yield?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
Hydrogen cyanide is produced industrially from the reaction of gaseous ammonia, oxygen, and methane:...
Questions

Social Studies, 03.11.2020 22:20


English, 03.11.2020 22:20

Mathematics, 03.11.2020 22:20





History, 03.11.2020 22:20


History, 03.11.2020 22:20

Mathematics, 03.11.2020 22:20


Social Studies, 03.11.2020 22:20


Mathematics, 03.11.2020 22:20

Mathematics, 03.11.2020 22:20

Biology, 03.11.2020 22:20


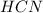 and 1124,69 g of
and 1124,69 g of 
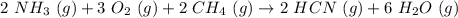
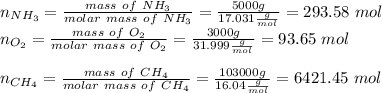
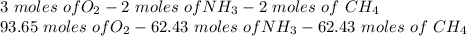
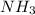 and
and 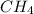 to consume the oxygen. In case there wasn´t enough of one of the other reactants, that one would be the limiting one.
to consume the oxygen. In case there wasn´t enough of one of the other reactants, that one would be the limiting one.