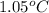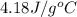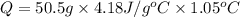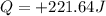Chemistry, 03.09.2019 01:30 ToriChristine
A0.500-g sample of kcl is added to 50.0g of water in a calprimeter (figure 5.12) if the temperature decreases by 1.05c. what is the approximate amount of heat involved in the dissolution of the kcl , assuming the heat capacity of the resulting solution is 4.18 j/gc? is the reaction exothermic or endothermic?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
A0.500-g sample of kcl is added to 50.0g of water in a calprimeter (figure 5.12) if the temperature...
Questions

Physics, 21.05.2020 05:00

Mathematics, 21.05.2020 05:00


Mathematics, 21.05.2020 05:00

Mathematics, 21.05.2020 05:00









English, 21.05.2020 05:00


History, 21.05.2020 05:00




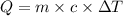
 = change in temperature =
= change in temperature =