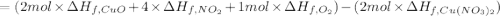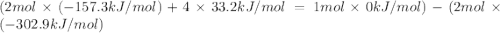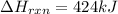The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) + 4no2(g) + o2(g) calculate the enthalpy change for this reaction using the following enthalpy changes of formation. ah! [cu(no3)2) = -302.9 kj mol? ah, (cuo) = -157.3 kj mol? . ah[no2) = +33.2 kj mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) +...
Questions

Mathematics, 21.10.2020 21:01

Social Studies, 21.10.2020 21:01

Business, 21.10.2020 21:01


Health, 21.10.2020 21:01

Biology, 21.10.2020 21:01

History, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01



Social Studies, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01


Mathematics, 21.10.2020 21:01

Chemistry, 21.10.2020 21:01


Chemistry, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

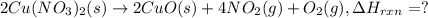
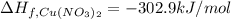
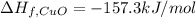
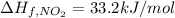
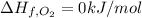 (standard state)
(standard state)![\Delta H_{rxn}=\sum [\Delta H_f(product)]-\sum [\Delta H_f(reactant)]](/tpl/images/0221/5790/84aad.png)
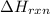 =
=