
Chemistry, 03.09.2019 03:30 prohrer589
Calculate ho298 for the process
sb + 5/2cl2 --> sbcl5
from the following information:
sb + 3/2cl2 --> sbcl3 ho298 = -314kj
sbcl3 + cl2 --> sbcl5 ho298 = -80kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Calculate ho298 for the process
sb + 5/2cl2 --> sbcl5
from the following information...
sb + 5/2cl2 --> sbcl5
from the following information...
Questions








Chemistry, 15.07.2019 00:00

Mathematics, 15.07.2019 00:00


History, 15.07.2019 00:00






History, 15.07.2019 00:00



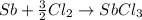
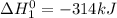 ..........(1)
..........(1)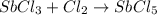
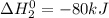 ..............(2)
..............(2)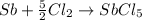
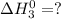 .............(3)
.............(3)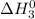 at 298 K will be as follows.
at 298 K will be as follows.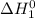 +
+ 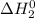
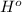 at 298 K for the given process is -394 kJ.
at 298 K for the given process is -394 kJ.

