
Chemistry, 03.09.2019 03:30 tilly40oooo
The half-life for the second-order decomposition of hi is 15.4 s when the initial concentration of hi is 0.67 m. what is the rate constant for this reaction? a) 1.0 * 10-2 m-15-1 b) 4.5 * 10-2 m-15-1 c) 9.7* 10-2 m-15-1 od) 2.2 * 10-2 m-15-1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
The half-life for the second-order decomposition of hi is 15.4 s when the initial concentration of h...
Questions



Mathematics, 23.03.2021 19:50

English, 23.03.2021 19:50



Social Studies, 23.03.2021 19:50

English, 23.03.2021 19:50


Social Studies, 23.03.2021 19:50

Mathematics, 23.03.2021 19:50

Chemistry, 23.03.2021 19:50

Mathematics, 23.03.2021 19:50


Physics, 23.03.2021 19:50

Mathematics, 23.03.2021 19:50



Mathematics, 23.03.2021 19:50

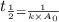
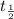 = half life = 15.4 s
= half life = 15.4 s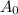 = initial concentration = 0.67 M
= initial concentration = 0.67 M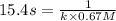
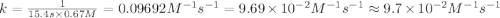
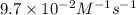 is the rate constant for this reaction.
is the rate constant for this reaction.

